Mendeleev's Periodic Law and Periodic Table
D'mitri Mendeleev, a Russian chemist, studied the properties of all the 63 elements known at that time and their compounds. On arranging the elements in the increasing order of atomic masses, he observed that the elements with similar properties occur periodically.
In 1869, he stated this observation in the form of the following statement which is known as the Mendeleev’s Periodic Law. The chemical and physical properties of elements are a periodic function of their atomic masses.
A periodic function is the one which repeats itself after a certain interval. Mendeleev arranged the elements in the form of a table which is known as the Mendeleev’s Periodic Table.
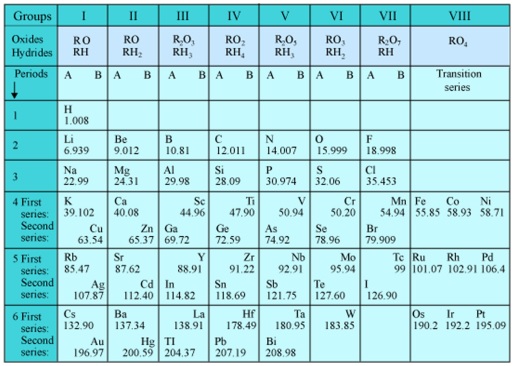
Mendeleev’s Periodic Table
Mendeleev arranged the elements in the increasing order of their atomic masses in horizontal rows till he came across an element whose properties were similar to those of the first element. Then he placed this element below the first element and thus started the second row of elements.
The success of Mendeleev’s classification was due to the fact that he laid more emphasis on the properties of elements rather than on atomic masses. Occasionally, he could not find an element that would fit in a particular position. He left such positions vacant for the elements that were yet to be discovered. He even predicted the properties of such elements and of some of their compounds fairly accurately.
In some cases, he even reversed the order of some elements, if it better matched their properties. Proceeding in this manner, he could arrange all the known elements in his periodic table.
When more elements were discovered, this periodic table was modified and updated to include them. One more group (zero group) had to be added when noble gases were discovered.
Main Features of Mendeleev’s Periodic Table
-
The elements are arranged in rows and columns in the periodic table.
-
The horizontal rows are called periods. There are six periods in the periodic table. These are numbered from 1 to 6 (Arabic numerals). Each one of the 4th, 5th and 6th periods have two series of elements.
-
Properties of elements in a given period show regular gradation (i.e. increase or decrease) from left to right.
-
The vertical columns present in it are called groups. There are eight groups numbered from I to VIII (Roman numerals).
-
Groups I to VII are further divided into A and B subgroups. However, group VIII contains three elements in each of the three periods.
-
All the elements present in a particular group are chemically similar in nature. They also show a regular gradation in their physical and chemical properties from top to bottom.
Merits of Mendeleev’s Periodic Classification
1. Classification of all elements
Mendeleev’s classification included all the 63 elements known at that time on the basis of their atomic mass and facilitated systematic study of elements.
2. Correction of atomic masses
Atomic masses of some elements like Be (beryllium), Au (gold), In (indium) were corrected based on their positions in the table.
3. Prediction of new elements
Mendeleev arranged the elements in the periodic table in increasing order of atomic mass but whenever he could not find out an element with expected properties, he left a blank space. He left this space blank for an element yet to be discovered. He even predicted the properties of such elements and also of some of their compounds.
For example, he predicted the existence of unknown element for the vacant space below silicon and thus belonging to the same group IV B, of the periodic table. He called it eka-silicon (meaning, one position below silicon). Later, in 1886, C.A. Winkler of Germany discovered this element and named it as germanium. The predicted and the actual properties of this element were remarkably similar.
Ekaboron (scandium) and eka-aluminium (gallium) are two more examples of unknown elements predicted by Mendeleev.
4. Valency of elements
Mendeleev’s classification helped in understanding the valency of elements. The valency of elements is given by the group number. For example, all the elements in group 1 i.e. lithium, hydrogen, sodium, potassium, rubidium, caesium have valency 1.
Defects of Mendeleev’s Periodic Table
Mendeleev’s periodic table was a great success, yet it had the defects.
1. Position of Hydrogen
The position of hydrogen which is placed in group IA along with alkali metals is ambiguous as it resembles alkali metals as well as halogens (group VII A).
2. Position of Isotopes
All the isotopes of an element have different atomic masses therefore, each one of them should have been assigned a separate position. On the other hand, they are all chemically similar; hence they should all be placed at the same position.
In fact, Mendeleev’s periodic table did not provide any space for different isotopes. For example, two isotopes of carbon are represented as 6C12, 6C14 but placed at the same position.
3. Anomalous Pairs of Elements
At some places, an element with greater atomic mass had been placed before an element with lower atomic mass due to their properties. For example, cobalt with higher atomic mass (58.9) was placed before nickel with lower atomic mass (58.7).
Other such pairs are: (i) Tellurium (127.6) is placed before iodine (126.9) and (ii) Argon (39.9) is placed before potassium (39.1).
4. Grouping of chemically dissimilar elements
Elements such as copper and silver have no resemblance with alkali metals (lithium, sodium, etc.), but have been grouped together in the first group.
5. Separation of chemically similar elements
Elements which are chemically similar such as gold and platinum have been placed in separate groups.