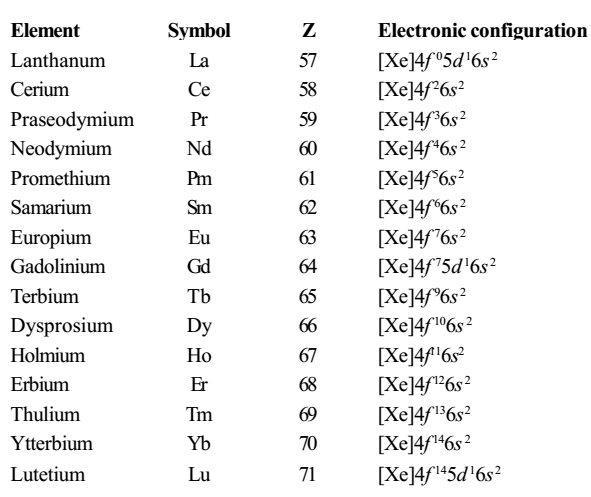
Electronic Configuration of f-Block Elements
Lanthanum is the first member of the third transition series, and it has one 5d and two 6s electrons. The next element is cerium, which while still retaining two 6s electrons, has two electrons in the 4f orbitals and none in the 5d orbitals. There are 7 separate 4f orbitals, each of which can accommodate two electrons with opposite spins.
The atoms of the elements from cerium to lutetium have two to fourteen electrons in 4f- orbitals, respectively. These elements constitute the first inner transition series known as lanthanides and, although lanthanum itself does not possess any 4f electrons, it is customary to include this element in this series.