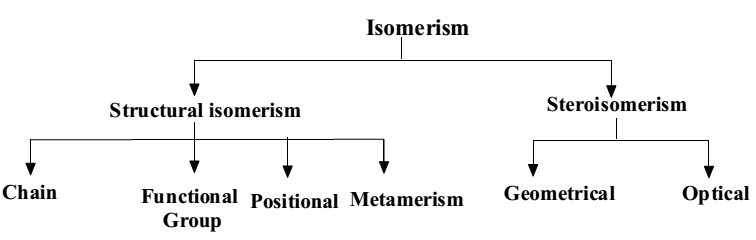
Isomerism
The simple alkanes containing upto three carbon atoms (methane, ethane and propane) have only one possible structure. There is only one way in which the carbon atoms can be linked together.
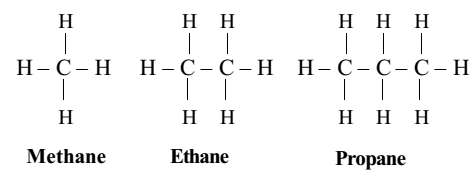
But for next higher hydrocarbon (butane, C4H10), there are two possible ways in which the carbon atoms can be linked together. They may be linked to form a straight chain or a branched chain.
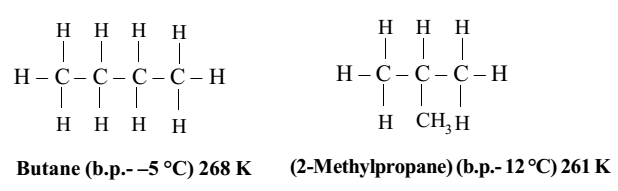
Thus, there are two types of butane which are different compounds and they show different properties.
Different substances which have the same molecular formula but differ in their structures, physical or chemical properties are called isomers and this phenomenon is known as isomerism.
Types of Isomerism