Langmuir Adsorption Isotherm
One of the drawbacks of the Freundlich adsorption isotherm is that it fails at high pressure of the gas. Langmuir derived an adsorption isotherm on theoretical considerations based on kinetic theory of gases.
This isotherm is based on the assumption that every adsorption site is equivalent and that the ability of a particle to bind there is independent of whether or not nearby sites are occupied. In his derivation, Langmuir considered adsorption to consist of the following two opposing processes:
- Asorption of the gas molecules on the surface of the solid
- Desorption of the adsorbed molecules from the surface of the solid
Langmuir believed that eventually a dynamic equilibrium is established between the above two opposing processes. He also assumed that the layer of the adsorbed gas is only one molecule thick (unimolecular). Since such type of adsorption is obtained in the case of chemisorption, Langmuir adsorption isotherm works particularly well for chemisorption.
The Langmuir adsorption isotherm is represented by the relation:
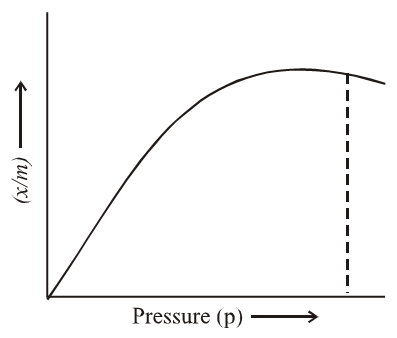
x/m = ap/(1+bp)
where a and b are two Langmuir parameters. At very high pressure, the isotherm acquires the limiting form.
x/m = a/b (at very high pressure)
At very low pressure,
x/m = ap (at very low pressure)
A plot of m/x against 1/p gives a straight line the slope and intercept equal to 1/a and b/a, respectively. Thus, both parameters can be determined.